Stories from the University of Cambridge
A low-cost and open source solution to automate imaging and analysis of cyst nematode infection assays for Arabidopsis thaliana
-
Olaf Prosper Kranse[1], Itsuhiro Ko[1],[2], Roberta Healey[1], Unnati Sonawala1, Siyuan Wei[1], Beatrice Senatori1, Francesco De Batté[1], Ji Zhou[3],[4], Lawrence Percival-Alwyn[4], Sebastian Eves-van den Akker[1]
1 Department of Plant Sciences, The Crop Science Centre, University of Cambridge, Cambridge, UK 2 Plant Pathology Department, Washington State University, Pullman, WA, USA 3 Jiangsu Collaborative Innovation Center for Modern Crop Production Co-Sponsored By Province and Ministry, Academy for Advanced Interdisciplinary Studies, Nanjing Agricultural University, Nanjing, China 4 Cambridge Crop Research, National Institute of Agricultural Botany (NIAB), Cambridge, UK
-
2022
-
Olaf Kranse:
ok297@cam.ac.ukSebastian Eves-van den Akker: se389@cam.ac.uk
-
Kranse OP, Ko I, Healey R, Sonowala U, Wei S, Senatori B, De Batté F, Zhou J, Eves-van den Akker S. 2022. A low-cost and open source solution to automate imaging and analysis of cyst nematode infection assays for Arabidopsis thaliana. Plant Methods 18, 134.
-
https://github.com/OlafKranse/A_low_cost_imaging_tower
-
Biomaker (UK), Biotechnology and Biological Sciences Research Council (UK), Department for Environment Food and Rural Affairs (UK, Frank Smart Scholarship (UK), Ministry of Science and Technology of the People’s Republic of China (China)
ABOUT THE OPEN-RESOURCE
Background
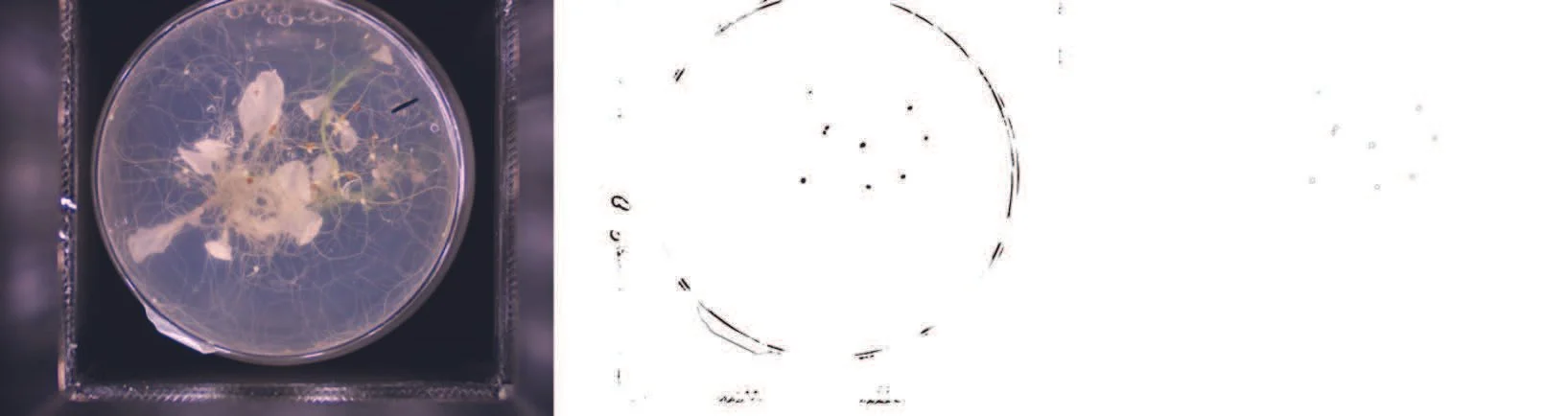
Plant-parasitic nematodes are responsible for considerable losses in crop yield. However, the current screening methods used to phenotype nematode infections are slow, limiting the knowledge that can be generated in the plant pathology field. “It all started off as a side project, but then when we realised the potential output of the machinery, it has become my main focus,” says Olaf Kranse, a postgraduate student in the Plant-Parasite/Pathogen Interactions group, at the Crop Science Centre. To study nematode parasitism, the group utilises the ability of Heterodera schachtii (the beet cyst nematode) to infect the model plant Arabidopsis thaliana. Olaf’s focus is to determine the frequency of infection for various host genotypes. While analysing some Arabidopsis mutant lines as part of his PhD project, Olaf realised that some of the plants had reduced root area, which could be an important factor for infection. The challenge would be to normalise infection to the root surface areas in a quick way, as manual measurement of the root surface area is extremely time consuming. While Olaf was playing with some of the root images, he noticed that it was possible to see the nematodes clearly on these plates. “And if you could also automatically count the nematodes? Problem solved,” says Olaf. The outcome is a system that allows the user to access root surface area, and nematode numbers and sizes. Previously the nematodes were counted manually using a microscope, which is a very low throughput technique. In Olaf’s opinion, he was lucky that nobody else developed a similar tool before, as it has probably been doable for a long time.
Function
This open source project is by design a low-cost easy-to-build and -use image analysis system to measure root area and phenotype nematode infection. But it does more: essentially, it can be used to image anything that fits in a 5 cm Petri dish, i.e., it could potentially be used for measuring plant growth over time, or any other system that fits in the tool.
Development process
In the initial development stages, the imaging system was a desk lamp with a camera taped to it, to assess the possibility of capturing a photo of a plate. As Olaf and the research group saw potential in the rudimentary system, the next step was to design a more complex imaging system, consisting of a lens mounted to a camera, which is connected to a Raspberry Pi (a small, affordable computer). These imaging components are then assembled onto a custom 3D-printed “tower”. Even though Olaf had some experience with 3D printing before, designing pieces that slot into each other perfectly showed to be challenging as the printing system has imperfections. Another challenge faced by the developers was related to the knowledge needed in the engineering field. A good lesson learned by Olaf was to get more people involved in the development process, as an extra person looking into it with a different perspective can save a lot of time and bring new ideas.
Target user
As this imaging system is optimised for small plants (i.e. Arabidopsis), it potentially limits its application to researchers working on other plant systems. The target user is anyone who needs consistent images of tissue culture in 5 cm petri dishes.
Comparison to other technologies
The use of imaging analysis systems is becoming more popular in the biological sciences field. Yet, for plant parasitic nematode studies, this is the only one available at the moment. Olaf presented this tool in 2021 at a nematology conference and the feedback was very positive “people said, finally, we don’t have to count nematodes under the microscope anymore, because it takes a lot of time to do this.”
IMPACT
Current use
As pointed out before, the imaging system is optimised for Arabidopsis. Currently, just the Plant-Parasite/Pathogen Interactions group at the University of Cambridge is using it, but more researchers in the area are getting interested in it. Olaf and his colleagues are thinking about future versions of the machine that could be used in crops, opening its use to crop research too.
Open source choice
There were different reasons why Olaf and his colleagues decided to make the imaging system open source. “The entire idea behind this was to make it affordable because in my opinion it’s important that any member of the academic community has access to these tools.” On the other hand, Olaf points out that there is the argument that if a technology is commercialised, the throughput and outreach are potentially higher.
GOING FORWARD - WHERE TO IN THE NEXT 3-5 YEARS?
Now that the Plant-Parasite/Pathogen Interactions group found an efficient way to take images of roots and nematodes, the next, bigger bottleneck is analysing the images, as some manual verification is required. Machine learning approaches may be the next step to increase screening speed. Furthermore, the group strives to expand the use of the imaging system to crop systems, allowing those studying crop parasitism to be able to use the platform.
Example of an image taken by the imaging system, evidencing the nematodes (top) and the plant roots (bottom). © 2022, The Plant-Parasite/Pathogen Interactions group, licensed under CC-BY 4.0 (individual, open license).
“In my opinion it’s important that any member of the academic community has access to these tools.”
Olaf Prosper Kranse